Human brain theory
ISBN 978-3-00-068559-0
Monograph of Dr. rer. nat. Andreas Heinrich Malczan
5 The basal ganglia - a delay module
In addition to the downward-projecting neurons of class 5, the downward-projecting neurons of class 6 already existed in the old rope ladder system. While the axons of the class 5 neurons pulled downward on the motor side, the axons of the mean neurons of class 6 could pull downward on both the sensory and the motor side, because they were present in both motor and sensory nuclei.
Similarly, there were the class 4 neurons that could receive the ascending input from below, but the class 1 neurons could also do this. While the class 4 neurons existed only on the sensory side, the class 1 neurons existed on both sides, sensory and motor.
Class 6 and class 1 neurons belong to the mean system and are also found in all vertebrates today. The basal ganglia system developed from the mean system.
The basal ganglia are an important subsystem of the brain, about whose function and mode of action many different views are spread. The explanation of their signal-processing function in the current literature needs urgent correction!
The central structure of the basal ganglia is the dopaminergic substantia nigra pars compacta and the associated area tegmentalis centralis (VTA). Together, they are the dopaminergic median nucleus of the brain. Originally, there was probably also this mean nucleus in each segment, but due to neuronal competition via lateral inhibition, only the nucleus in the seventh segment remained, which is where all the signals from the trunk arrived.
The fact that all sensory and motor nuclei received a back projection from the mean nuclei contributed to the development. Initially, the projection was always limited to one segment, but the mean nuclei also crossed the segment boundaries with their signals. This was the beginning of the neuronal competition, which only ended when one mean system (per transmitter) had gained supremacy and the corresponding mean centres regressed in the remaining segments. In the first six segments, the dependent receptors prevailed and displaced the other receptor types, but also most of the mean nuclei. An exception is the first segment, which as the oldest system - from which all segmented organisms descend - was very conservative and could often resist further changes. Due to the unfolding of the modalities, it is located in the temporal loop, the later temporal lobe.
Thus, in the seventh segment, the dopaminergic midline centre (among others) remained. The area tegmentalis centralis is the part of this mean nucleus that receives input from the olfactory, later limbic system. The substantia nigra pars compacta, on the other hand, receives the remaining input. It is likely that neuronal competition led to atrophy of the dopaminergic midline centres in the trunk segments. This would still have to be examined.
The specialisation of the seventh segment on a dopaminergic midline centre resulted in each head segment sending the signals of its sensory and motor nuclei to this dopaminergic midline centre via class 6 neurons. However, we should bear in mind that these neurons received their input in sensory nuclei and sensory cortex from layer 3. In motor nuclei and motor cortex, class 5 neurons were the input providers to class 6 neurons, the latter projecting to the seventh segment dopaminergic centre. Apparently, they were controlled by a marker that diffused from this centre into the environment. Neuron class 5, on the other hand, apparently follows a motor marker that is also segment-specific. It is very difficult to distinguish between the axons of these two classes, as they both tract downwards.
The olfactory or limbic signals of the first segment reach the VTA, the other segments all project into the substantia nigra pars compacta, which is directly adjacent. It is nevertheless well-ordered according to modalities, since each head segment processed other modalities. We therefore assume that the dopaminergic nucleus was also ordered by modality. This is also recognisable from the fact that different lobes of the brain project into different sections of this nucleus.
Specifically, the first segment, the later cortex, also projected with all non-olfactory signals into the seventh segment to the substantia nigra pars compacta.
Now each mean nucleus projects back to the location of its signal origin. Therefore, there is a back projection of the substantia nigra pars compacta and the VTA in the direction of the signal origin. However, as cytoarchitectonic studies have shown, this usually ends at the inhibitory interneurons of the target structures that provided the original signals.
What changes in response behaviour did the back projection bring about?
It was the time difference. The returning signals were time-delayed. If nothing had changed in the meantime, the output was inhibited at its own rate of fire. Only if something changed - i.e. moved - did a residual signal remain, but only if the (exciting) present signal was stronger than the inhibiting past signal. In this way, movements could be detected visually, smelled, felt. Even self-movements became perceptible. The inhibitory back projection established the so-called matrix in the striatum of the basal ganglia. Its axons are inhibited by dopamine.
However, there was also - and much earlier - an excitatory back-projection from the dopaminergic mid-segment nucleus of the seventh segment.
The excitatory dopaminergic projection was to the striosomes that initially made up the basal ganglia. They projected inhibitory into the nucleus ruber and produced a time-sensitive differential mapping there for movement analysis. Their origin and function were described in my previous monograph and are skipped here.
The inhibitory back projection formed in later evolutionary time. Initially, it inhibited signal output in the cortex with a time delay if nothing had changed in the meantime.
However, since it was more favourable if the previous excitatory projection was maintained and triggered the previous reactions in the spinal cord, at some point the differential signal was no longer formed in the cortex, but led to the formation of independent projection neurons. Therefore, the basal ganglia are a nucleus with its own output.
How did this come about?
The inhibitory dopaminergic back-projection became specific over time. Each cortex neuron, each output neuron of a sensory or motor nucleus in segments 1 to 6 projected into exactly one neuron of the substantia nigra pars compacta. This neuron switched the transmitter from glutamate to dopamine and sent its axon to the origin structure of the signal. There, its axon docked onto an inhibitory interneuron.
These inhibitory interneurons, which received the dopaminergic input from the substantia nigra pars compacta or from the VTA, separated spatially from the original cortical structure and formed their own nucleus. In the first, cortical segment, the striatum thus emerged below the cortex cortex. Its inhibitory projection neurons are descendants of the inhibitory interneurons of the cortex. The inhibitory output of this new nucleus could now be superimposed on the original cortex signals and the signals of the other segments. This superimposition took place partly in the nucleus ruber, through which all the motor output of the primordial brain passed to the motor neurons of the trunk for the purpose of motor control of the body. Part of the striatal neurons - more precisely, the striosomal neurons - projected inhibitively into the nucleus ruber. These axons are an important part of the tractus tegmentalis centralis.
The GABA-egen matrix neurons of the striatum projected downwards and reached (on the motor side) the motor nucleus of the second floor, which itself received a complete copy of the sensory nucleus of this floor delivered by the class 3 neurons, which was excitatory. There, these signals overlapped in a point-to-point mapping. While the sensory nucleus of this floor became the sensory thalamus, the motor nucleus developed into the motor (ventral) thalamus.
Those signals from the sensory thalamus that projected to the sensory cortex areas then passed through the basal ganglia and, after passing through the basal ganglia, returned to the motor thalamus in an inhibitory manner. The latter received the same, but excitatory input from the sensory thalamus via the neurons of class 3. The point-by-point superimposition of both types of signals produced a time-sensitive differential image that responded to movements. This was delivered back to the cortex where it reached new association areas for movement and direction recognition. The motor thalamus corresponds to the pulvinar for visual modalities. It receives a complete excitatory copy of the primary visual thalamus via collaterals, which is overlaid with the inhibitory striatal signals and produces a time-sensitive differential mapping that recognises movements of a visual nature. The output reaches new, secondary cortical association areas that are used to analyse movement and location.
Thus there were overlapping areas in the motor thalamus. We will call it the secondary thalamus. The primary thalamus - like the primary cortex (with the exception of the frontal cortex) - receives input from receptors.
The dopaminergic signals were time-delayed compared to the original signals from which they were obtained by transmitter switching. The diversions via the substantia nigra pars compacta or the VTA had delayed them by several milliseconds. After switching to GABA, they reached the target structures in the secondary cortex with a certain time delay in order to overlap with the original signals.
This made movement detection possible. If nothing had changed in the meantime, the inhibitory past signal from the striatum cancelled out the excitatory, undelayed present signal. However, if the excitatory present signal had increased in strength, the old past signal, which had arisen several milliseconds earlier, could no longer completely extinguish it. A residual signal remained, the strength of which encoded the signal change. It reached the motor neurons via the nucleus ruber and triggered reactions that were previously impossible.
The basal ganglia served to detect movement. Wherever an object, e.g. a prey animal, was moving, its movement could be smelled, felt, detected by the lateral line sense and seen by means of the basal ganglia. Every modality was involved, because every head segment and every trunk segment sent its signals towards the cortex and into the seven head segments, where the movement analysis could even be analysed separately by modality.
And because it was cheaper to keep the original, uninhibited signals as well, these original signals were split. A complete copy of the signals reached a new thalamic nucleus, which is also called the ventral thalamus. There, a complete copy of the cortex signals is available. Class 3 neurons are used for this purpose. They project from the sensory to the motor side in each segment. Since the same signals ascend to the cortex, also change from the sensory to the motor cortex side and descend again, they pass this ventral thalamus again. The time-delayed and inhibitory signals from the matrix of the striatum also enter there and erase all the present signals that had not changed in the meantime. Only movements or changes in signal strength remained.
Thus, this nucleus possessed the time-sensitive differential mapping and could in turn send it excitatory to the cortex for further evaluation.
Since the functioning of the basal ganglia is mainly based on the inhibition of mean value signals, i.e. it ultimately represents a signal inversion, a monotonicity reversal is also realised here. However, a maximum-encoded signal is minimum-encoded by a monotonicity inversion. Therefore, a second signal inversion is necessary. This is realised by the globus pallidus. This causes the inverted output to be maximum-encoded again. In the basal ganglia, every maximum-encoded signal is thus inverted twice, once in the matrix of the striatum and once in the globus pallidus. This is a very strong indication of the correctness of my theory of extreme value coding and signal inversion.
For the motor area, the circuitry of the basal ganglia is shown in the following figure of mine.
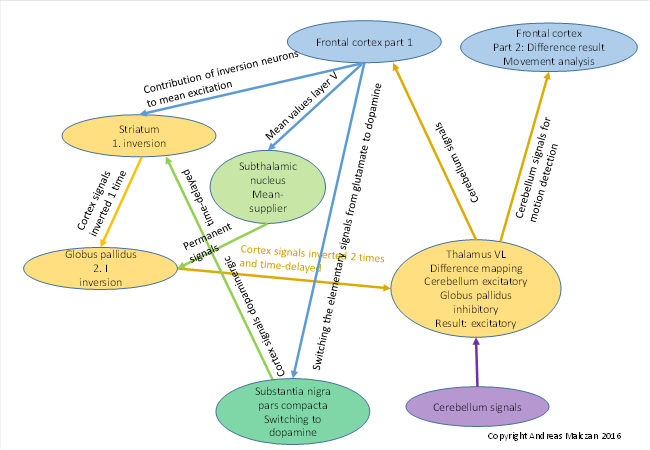
Figure 37: Basal ganglia module and its circuitry for motor signals
I have described the gradual development of the basal ganglia in detail in my monograph "Brain Theory of Vertebrates". It is time for our leading neurologists to separate themselves from the outdated and incorrect views on the basal ganglia that still haunt all neurological textbooks today. In particular, I recommend studying the division of the striatum into the striosomes and the matrix, which is detailed in my previous monograph. Here the current view of the functioning of the basal ganglia must be completely reconsidered.
Now three important developments in the vertebrate brain are still missing. These are
1. The myelination of the projection axons
2. The splitting of modalities
3. The signal divergence
It would be idle to write about the importance of the formation of myelin for neuronal signal transmission.
Therefore, the description of the delay module for motion vision in the light-dark format now follows. The circuitry of the delay module is identical for all modalities, so we will deal here with the visual detection of movement by the light-dark module as an example. It allows the motion analysis of objects analysed by the light-dark module of the visual cortex. The visual colour modules work according to the same principle.
5.1 The visual delay module of the basal ganglia
In chapter 4.2.5. on the brightness module with spatial signal propagation, we had investigated how orientation columns in the visual cortex made it possible to encode the angle of rise of a bright straight line and its zero point distance via maximum coding. This was an important preliminary step for the shape recognition of dark objects, because the recognised line elements made it possible to recognise the outline of a dark object. The same principle was used to recognise bright objects. Subsequently, the analysis principle was transferred to the brightness modules and the colour modules of the human brain.
Four neighbouring retinal and magnocellular ganglion cells of each eye supply (via the visual thalamus) a cube-shaped light-dark module in the layer system S4-dark-on//S3-dark-on//S4-bright-on. They form a square - but only theoretically. Likewise, they could form a rhombus with each side having a different length. The mathematical principle of forming extremes by superimposing concave functions remains even then.
Likewise, four parvocellular ganglion cells of the blue type arranged at the same retinal location supply the input layer S4-blue arranged above it. This is followed by an output layer S3-blue, which, however, can also accept input from the input layer S4-green arranged above it. Above the layer S4-green follows the outpu layer S3-green, which also receives input from the input layer S4-red above it. Above S4-Red is the output layer S3-Red, which can also accept input from the input layer S4-Blue above it. The topmost outpu layer S3-Blue was able to degenerate in the course of evolution; it became superfluous through the development of signal overlay.
Thus, there were six quite thin input layers in this cuboid cortex module, two for light-on and light-off, two for the colour input red and green as well as two layers for the colour blue, the latter one lower and one upper layer each.
And there was one outpour layer for the signal type brightness and three for the mixed colours blue/green, green/red and red/blue.
Each of these orientation columns, which analysed a retinal square in terms of brightness and colour, had many thousands or hundreds of thousands of output neurons in its total of four quite thick output layers.
We learned that the outpour layers 3 - even if they had "disintegrated" into sub-layers, passed on their output via two directions. Upwards there was layer 2 - which then (probably) also formed sublayers associated with the type 3 sublayers. They projected to other cortex areas.
Likewise, there was signal transport downwards. Initially, layer 6 took over the signals from layer 3 and supplied the midline structures in the seventh segment. Thus, the dopaminergic substantia nigra also received these signals.
However, when the number of neurons increased greatly in the type 3 layers and this layer and the sub-layers that emerged from it became wider and thicker, it did not make sense to have these signals summarised by large mean signals. It was much more favourable if these signals could travel unaltered to the substantia nigra. There they were switched to the transmitter dopamine. Now they moved back to the matrix of the striatum. There they were switched to GABA and the signals were sent back to the ventral thalamus.
However, the output of the primary visual cortex also arrived there. This is because it projected into secondary fields on the motor side of the occipital lobe. Its descending signals also passed through this ventral thalamus.
Thus, two types of signals were available in the ventral thalamus for the entire primary visual cortex:
- Each output signal from each orientation column ended at its own neuron. It was exciting.
- Each of these signals also reached the ventral thalamus with a time delay via the pathway substantia nigra pars compacta-matrix of the striatum. It was inhibitory.
Thus, for each individual output neuron in the ventral thalamus, there was the excitatory present signal and the inhibitory past signal derived from it. Both ended at exactly one output neuron of the ventral thalamus. The output of this superimposing neuron only occurred when the past signal could not completely cancel out the present signal.
Since each output neuron belonged to exactly one orientation column, the past signal could only cancel out the present signal if both were equally strong. Then neither the brightness nor the colour in the associated retinal square was allowed to change. Strictly speaking, nothing was allowed to change there.
However, if the colour in this retinal square changed because a different coloured object became visible, or if the brightness changed because an object with a different brightness appeared, this specific output cell no longer fired. Because in all visual modules, the neuron with the highest firing rate was suddenly at a different height if the colour or brightness changed. The new output therefore had to be weaker.
When an inclined straight line was projected onto the retina in the associated retinal square, and this changed its angle of attack or its zero point approach (to the centre of the square), the location of maximum excitation moved from the current orientation column to another that was now firing most strongly. The firing rate of the current neuron became weaker, the present signal was weaker than the time-delayed past signal. Here, the residual signal was zero.
But there was a new output signal, because now the orientation column, which had been silent before, fired. Its past signal was the null signal and could not extinguish the new present signal. It was precisely this new orientation column that now fired its output uninhibited into the secondary areas of the cortex.
So, in that secondary visual cortex that receives visual output from the ventral thalamus, strongly firing neurons indicate movement and change. But these neurons have different visual submodalities: Light/Dark, Blue/Green, Green/Red and Red/Blue.
Different modalities, however, have the tendency to split up, to split, to rearrange themselves in the course of evolution. Therefore, in the secondary cortex that analyses movements, there is also a splitting according to these submodalities.
In particular, one should consider that (in my opinion) the thickness growth of the cortex cortex occurred before the expansion in width. It could even be that in reptiles there was no width growth at all and in birds only very little. Thus their cortex areas would be predominantly divergence layers with vertical signal spread. I have already formulated this as a hypothesis in my previous monograph.
If the width expansion occurred evolutionarily much later than the thickness growth, the neurons gained or newly formed as a result may well be regarded as a separate modality. Then the results of vertical signal divergence would be a separate, older modality (blobs), and the results of width growth, i.e. the orientation columns or interblobs would also be an independent, but younger modality. These two different modalities would separate in the secondary cortex and occupy independent areas. This indeed seems to be the case, as the following considerations show.
The middle neurons of each cuboidal analysis module, indicated by the values x = 0 and y = 0, project into two areas: One representing the brightness of an object (when it completely fills the entire four magnocellular retinal ganglion cells) and one for colour (when that object also completely fills the four parvocellular retinal ganglion cells). Since there are only a few brightnesses and colours of this type of recognition for each retinal point, these neurons form a narrow long strip. The retina is stretched out rectangularly. Instead of one pixel, there are a number of brightness neurons and likewise of colour neurons next to each other. The retina becomes a rectangular image with a relatively small height. For example, if the retina has 500 pixels in height and 500 in width, there is a rectangle in this area with 500 neurons in width and 500 * 20 = 10000 brightness neurons in length. A colour-sensitive area then needs a rectangle of 500 neurons in width and 3500 in length for the seven rainbow colours. To accommodate 500 neurons in width, we do not need much space for the quite small cortex neurons. Therefore, we find narrow strips there that react to changes in brightness and colour.
It is different with the output of those orientation columns that react to line elements. Here the output is passed to the basal ganglia and comes back with a time delay to the ventral thalamus, where these past signals inhibit the present signals. Thus, these superimposition neurons indicate a change in the line elements both in angle and in brightness and colour.
If the retina consists of 500 times 500 pixels, there are 250,000 pixels in total. If there are 100 orientation columns for each pixel, 100 different angles can be recognised. In total there are 25000000, i.e. 25 million pixels.
Now there are four submodalities for each of these orientation columns: Light/dark, blue/green, green/red and red/blue. That is already 100 million variants. This has to be multiplied by the number of brightness levels and colour variants. This number is huge.
Therefore, there is at least one broad strip of neurons that indicate the light-dark movement of contours (colour-neutral directional elements). Likewise, there is an even much wider strip that signals the colour of contour movements. If these neurons are arranged in one direction as they are in the colour module in the vertical direction, this "colour stripe" is much wider than the light-dark stripe.
In total, there are three strips of different widths, which receive their input partly via the diversions of the basal ganglia and which are sensitive to movement.
In the case of the orientation columns, the movement signal via action potential sequences is strongest when a line element is moved parallel in the direction that forms a right angle with the line element. However, if the line element moves along the line it forms itself, the associated orientation column reacts in two different possible ways:
- The line is so long that it still covers the ganglion cells involved just as it did before they moved. Then the "movement orientation column" does not fire: nothing has changed.
- If the line length is not sufficient to cover the ganglion cells after the movement, the cover suddenly falls away. The neuron fires strongly, indicating to us: End of line reached, line leaves the ganglion cell, change detected.
The latter property is called end inhibition. All neurons that receive input from the basal ganglia and lie in the broad strip of directionally sensitive neurons respond when the end of a line is reached by moving a line in its own line direction, thereby removing the occlusion of the ganglion cells.
The basal ganglia enable specific motion and direction recognition in all modalities. Their output reaches selected areas in the thalamus where it is overlaid with a copy of the original signals. The output of the differential mapping reaches secondary areas, which is used for motion and direction analysis of objects of any modality.
Since in the case of orientation columns not only the change in the angle of line elements is recognised, but there is even a particularly preferred direction of movement during the analysis, the brain can also determine the change in location from this information. It enables the perception that something has moved. However, it can also determine the direction of movement. But for this, it must be able to store the direction of movement determined during the signal change and make it available again later. In my opinion, this signal storage takes place mainly in the cerebellum, but also partly in the hippocampus.
I have understood the storage in the hippocampus in terms of content, but I am still missing important information to describe the algorithm in an understandable way. However, it is predictable that there must be neuronal organisation in the hippocampus in hexagonal formations that serve to encode locations. These hexagonal neuronal formations are a tribute to the hexagonal focal modules, which in turn are a consequence of six vertebrate eye muscles. The multilevel mean system of the vertebrate brain is involved. Hexagonal populations of different sizes in the hippocampus probably correspond to different levels of the mean system, i.e. different "magnification levels".
This encoding is independent of the current orientation around space. The hippocampal "map" is anchored quasi immovably in the brain; only one's own location is marked in this map by firing neurons and therefore wanders back and forth with one's own movements. Perhaps some readers will enjoy analysing the basis of the encoding of spatial coordinates in the hippocampus together with me. An essential aspect here is hippocampal learning, i.e. imprinting via LTP and LTD, which proceeds in a similar way as in the pontocerebellum. Here, too, tetanic mean signals are the neuronal write command in the neuronal network.
Monograph of Dr. rer. nat. Andreas Heinrich Malczan